रसायनिक तत्त्व धलः इतिहास बयान Nomenclature रसायनिक चिंत मात्रा लिधंसा स्वयादिसँ पिनेया स्वापूत Navigation menuchemical elementThe Problem of the Soul in Aristotle's De animaHeaviest element made - againElements 116 and 118 Are DiscoveredBig Bang NucleosynthesisSynthesis of the elements in stars: forty years of progressElementymology & Elements MultidictSDV Nuclear Glossaryवेबइलेमेन्टस्केमिकूलकेमिकलइलेमेन्टलस एलामोस देय् प्रयोगशालाe
पुष्टि(साइटेसन) मदुगु च्वसुरसायनिक तत्त्व
अणुआणविक ल्याखंहाइड्रोजननाइट्रोजनकार्बोन२००७टेक्नोसियमप्रोमेथियमआइसोटोपयुर्यानियमथोरियमरसायनिक वस्तुन्युक्लियर रियाक्सनतत्त्वप्लेटोटेट्राहेड्रनअक्टाहेड्रनआइकोसाहेड्रनक्युबfour elementsEmpedoclesAristotlequintessenceGeberParacelsusJohann BecherRobert Boyleclassical elementsAntoine LavoisierlightJöns Jakob BerzeliusMendeleevperiodic tablesubstancechemicalHenry Moseleyisotopesallotropesmendeleviumhydrogenheliumnucleosynthesisnuclear fissionAs of 2006technetiumpromethiumastatinefranciumneptuniumplutoniumcaliforniumhalf-livesTechnetiumby nameby symbolby atomic numberby densityby melting pointby boiling pointIonization energies of the elementsperiodic tableकार्बोनआणविक न्युक्लियसआइसोटोपmass numbernucleonsrelative atomic massatomic mass unitnuclear binding energyisotopesradioactiveatomic numbersallotropesdiamondfullerenesstandard statethermochemistryenthalpy of formationEnglishromance languagesofficial namesInternational Union of Pure and Applied ChemistryLatin alphabetcaliforniumeinsteiniumrulecarbon-12uranium-235half lifeelement naming controversyNew WorldalchemistsJohn DaltonBerzeliusLatin alphabetLatinsodiumtungstenmercurypotassiumgoldleadantimonyroman numeralhalogenradicalyttriumligandhydrogendeuteriumtritiumहेभी वाटरBig Bangnucleosynthesisdeuteriumgalactic halosStellar nucleosynthesisSupernova nucleosynthesisintergalactic spacevisible universe
(function()var node=document.getElementById("mw-dismissablenotice-anonplace");if(node)node.outerHTML="u003Cdiv class="mw-dismissable-notice"u003Eu003Cdiv class="mw-dismissable-notice-close"u003E[u003Ca tabindex="0" role="button"u003Edismissu003C/au003E]u003C/divu003Eu003Cdiv class="mw-dismissable-notice-body"u003Eu003Cdiv id="localNotice" lang="new" dir="ltr"u003Eu003Cdiv style="font-size:110%;border:none;margin: 0;padding:.1em;color:#000"u003Enu003Cp style="text-align:center;"u003Enu003Cbu003Eu003Ca href="/wiki/%E0%A4%B5%E0%A4%BF%E0%A4%95%E0%A4%BF%E0%A4%AA%E0%A4%BF%E0%A4%A1%E0%A4%BF%E0%A4%AF%E0%A4%BE:Input_System" class="mw-redirect" title="विकिपिडिया:Input System"u003Eनेपालभाषा विकिपिडियाया देवनागरी इन्पुट व्यवस्थाu003C/au003Eu003C/bu003Enu003C/pu003Enu003C/divu003Eu003C/divu003Eu003C/divu003Eu003C/divu003E";());
रसायनिक तत्त्व
Jump to navigation
Jump to search
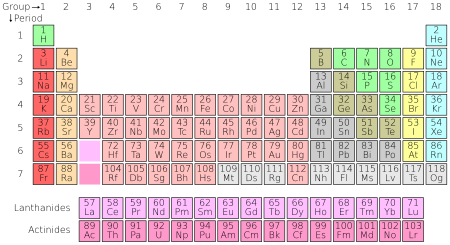
पिरियोडिक टेबलय् रसायनिक तत्त्वत
रसायनिक तत्त्व धागु छगु कथंया अणु ख। थन्यागु अणुतेत इमिगु आणविक ल्याखं नं परिभाषित याइ। आणविक ल्याखं धागु अणुया न्युक्लियसय् दुगु प्रोटोनया ल्या ख। थ्व खंग्व छगु हे प्रोटोन ल्या दुगु शुद्ध रसायनिक वस्तुयात इंगीय यायेत नं छ्येलि। [१]
तत्त्वया साधारण दसु हाइड्रोजन, नाइट्रोजन, कार्बोन आदि ख। सकलय् २००७ तक्कय् ११७ तत्त्वत लुयावगु दु। थुकिलि ९४ प्राकृतिक ख धासा आणविक ल्याखँ ८२ वा अप्व (बिस्मुथ व लिपाया तत्त्वत) मनुनं देकुगु ख। थ्व नापं, तत्त्व ४३ व ६१ (टेक्नोसियम व प्रोमेथियम)या स्थायी आइसोटोप मदु। थ्व निगु तत्त्व प्रकृतिय् ध्वगिना वनि। आणविक ल्याखं ९४ तक्कया तत्त्वतेगु स्थायी न्युक्लियस मदुसा थ्व ल्याखं तक्कया तत्त्वत प्रकृतिय् युर्यानियम व थोरियमया ध्वगीगु प्रकृयाय् प्राकृतिक तवलं दयाच्वनि। [२]
सकल रसायनिक वस्तुय् थ्व तत्त्वत दै। न्हुगु तत्त्वत इ-इले अप्राकृतिक न्युक्लियर रियाक्सननं बुया वया च्वंगु खने दु।
धलः
१ इतिहास
२ बयान
२.१ आणविक ल्याखँ
२.२ आणविक मात्रा
२.३ आइसोटोप
२.४ एलोट्रोफी
२.५ स्ट्यान्डर्ड स्टेट
३ Nomenclature
४ रसायनिक चिंत
४.१ विषेश रसायनिक तत्त्व
४.२ साधारण रसायनिक चिं
४.३ आइसोटोप चिंत
५ मात्रा
६ लिधंसा
७ स्वयादिसँ
८ पिनेया स्वापूत
८.१ रसायनिक सूचं
इतिहास
'तत्त्व' धागु खंग्व संस्कृतं वगु ख। पाश्चात्य हलिमे युनानी दार्शनिक प्लेटोनं ३६०य् छगु खंल्हाबंल्हा ज्याझ्वले इनर्ग्यानिक व अर्ग्यानिक तत्त्वतेगु बारेय् धयादिगु खं तत्त्वया बारेय् दक्ले पुलांगु मुनातगु दस्ताबेजय् छगु ख। प्लेटो कथं सकल तत्त्वया चिधंगु पार्टिकलया विशेष आकार दै : टेट्राहेड्रन (मि), अक्टाहेड्रन (वायु), आइकोसाहेड्रन (ल), व क्युब (पृथ्वी)[३]
 | 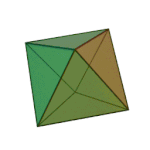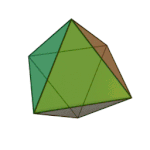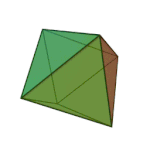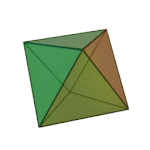 |  |  | |
टेट्राहेड्रन (मि) | अक्टाहेड्रन (वायु) | आइकोसाहेड्रन (ल) | क्युब (पृथ्वी) |
Adding to the four elements of the Greek philosopher Empedocles, in about 350 BC, Aristotle also used the term "element" and conceived of a fifth element called "quintessence", which formed the heavens. Aristotle defined an element as:
Element – one of those bodies into which other bodies can be decomposed and which itself is not capable of being divided into other.[४]
Building on this theory, in c. 790 Arabian chemist Jabir ibn-Hayyan (Geber) postulated that metals were formed out of two elements: sulphur, ‘the stone which burns’, which characterized the principle of combustibility, and mercury, which contained the idealized principle of metallic properties.[५] Shortly thereafter, this evolved into the Arabic concept of the three principles: sulphur giving flammability or combustion, mercury giving volatility and stability, and salt giving solidity.
In 1524, Swiss chemist Paracelsus adopted Aristotle’s four element theory, but reasoned that they appeared in bodies as Geber’s three principles. Paracelsus saw these principles as fundamental, and justified them by recourse to the description of how wood burns in fire. Mercury included the cohesive principle, so that when it left in smoke the wood fell apart. Smoke represented the volatility (the mercury principle), the heat-giving flames represented flammability (sulphur), and the remnant ash represented solidity (salt).[५]
In 1669, German physician and chemist Johann Becher published his Physica Subterranea, in which, in modification on the ideas of Paracelsus, he argued that the constituents of bodies are air, water, and three types of earth: terra fluida, the mercurial element, which contributes fluidity and volatility; terra lapida, the solidifying element, which produces fusibility or the binding quality; and terra pinguis, the fatty element, which gives material substance its oily and combustible qualities.[६] These three earths correspond with Geber’s three principles. A piece of wood, for example, according to Becher, is composed of ash and terra pinguis; when the wood is burnt, the terra pinguis is released, leaving the ash. In other words, in combustion the fatty earth burns away.
In 1661, Robert Boyle showed that there were more than just four classical elements as the ancients had assumed.[७] The first modern list of chemical elements was given in Antoine Lavoisier's 1789 Elements of Chemistry, which contained thirty-three elements, including light and caloric. By 1818, Jöns Jakob Berzelius had determined atomic weights for forty-five of the forty-nine accepted elements. In 1869, in Mendeleev's famous periodic table, shown below, there were sixty-six elements.

Mendeleev's 1869 periodic table
From Boyle until the early 20th century, an element was defined as a pure substance that cannot be decomposed into any simpler substance.[७] Said another way, an "element" cannot be transformed into other chemical substances by chemical processes. In 1913, Henry Moseley discovered that the physical basis of the atomic number of the atom was its nuclear charge, which eventually led to the current definition. The current definition also avoids some ambiguities due to isotopes and allotropes.
By 1919, there were seventy-two known elements.[८] In 1955, element 101 was discovered and named mendelevium in honor of Mendeleev, the first to arrange the elements in a periodic manner. In October 2006, the synthesis of element 118 was reported; however, element 117 has not yet been created in the laboratory.
बयान
The lightest elements are hydrogen and helium. All the heavier elements are made, both naturally and artificially, through various methods of nucleosynthesis, including occasionally nuclear fission.
As of 2006, there are 117 known elements (in this context, "known" means observed well enough, even from just a few decay products, to have been differentiated from any other element).[९][१०] Of these 117 elements, 94 occur naturally on Earth. Six of these occur in extreme trace quantities: technetium, atomic number 43; promethium, number 61; astatine, number 85; francium, number 87; neptunium, number 93; and plutonium, number 94. These 94 elements, and also possibly element 98 californium, have been detected in the universe at large, in the spectra of stars and also supernovae, where short-lived radioactive elements are newly being made.
The remaining 22 elements not found on Earth or in astronomical spectra have been derived artificially. All of the solely-artificially derived elements are radioactive with very short half-lives; if any atoms of these elements were present at the formation of Earth, they are extremely likely to have already decayed, and if present in novae, have are in quantities too small to have been noted. Technetium was the first purportedly non-naturally occurring element to be synthesized, in 1937, although trace amounts of technetium have since been found in nature, and the element may have been discovered naturally in 1925. This pattern of artificial production and later natural discovery has been repeated with several other radioactive naturally-occurring trace elements.
Lists of the elements by name, by symbol, by atomic number, by density, by melting point, and by boiling point as well as Ionization energies of the elements are available. The most convenient presentation of the elements is in the periodic table, which groups elements with similar chemical properties together.
आणविक ल्याखँ
छगु तत्त्वया आणविक ल्याखँ थुकिलि दैगु प्रोटोनया ल्या बराबर जुइ। दसु- सकल कार्बोन अणुया आणविक न्युक्लियसय् ६गः प्रोटोन दै, अथे जुगुलिं कार्बोनया आणविक ल्याखँ (Z)=६ ख। थन्यागु अणुय् न्युट्रोनया ल्याखं पाय्फु, थथे न्युट्रोनया ल्याखँ पागु अणुतेत आइसोटोप धाइ।
आणविक मात्रा
The mass number of an element, A, is the number of nucleons (protons and neutrons) in the atomic nucleus. Different isotopes of a given element are distinguished by their mass numbers, which are conventionally written as a super-index on the left hand side of the atomic symbol (e.g., 238U).
The relative atomic mass of an element is the average of the atomic masses of all the chemical element's isotopes as found in a particular environment, weighted by isotopic abundance, relative to the atomic mass unit (u). This number may be a fraction which is not close to a whole number, due to the averaging process. On the other hand, the atomic mass of a pure isotope is quite close to its mass number. Whereas the mass number is a natural (or whole) number, the atomic mass of a single isotope is a real number which is close to a natural number, which in general differs slightly from the mass number because the mass of the protons and neutrons is not exactly 1 u, because the electrons also contribute slightly to the atomic mass, and because of the nuclear binding energy. For example, the mass of 19F is 18.9984032 u. The only exception to the atomic mass of an isotope not being a natural number is 12C, which has a mass of exactly 12, due to the definition of u (it is fixed as 1/12th of the mass of a free carbon-12 atom, exactly).
आइसोटोप
Some isotopes are radioactive and decay into other elements upon radiating an alpha or beta particle. Certain elements have no nonradioactive isotopes: specifically the elements without any stable isotopes are technetium (atomic number 43), promethium (atomic number 61), and all observed elements with atomic numbers greater than 82.
एलोट्रोफी
Some elements can be found as multiple elementary substances, known as allotropes, which differ in their structure and properties. For example, carbon can be found as diamond, which has a tetrahedral structure around each carbon atom; graphite, which has layers of carbon atoms with a hexagonal structure, and fullerenes, which have nearly spherical shapes.
स्ट्यान्डर्ड स्टेट
The standard state, or reference state, of an element is defined as its thermodynamically most stable state at 1 bar at a given temperature (typically at 298.15 K). In thermochemistry, an element is defined to have an enthalpy of formation of zero in its standard state. For example, the reference state for carbon is graphite, because it is more stable than the other allotropes.
Nomenclature
The naming of elements precedes the atomic theory of matter, although at the time it was not known which chemicals were elements and which compounds. When it was learned, existing names (e.g., gold, mercury, iron) were kept in most countries, and national differences emerged over the names of elements either for convenience, linguistic niceties, or nationalism. For example, the Germans use "Wasserstoff" for "hydrogen" and "Sauerstoff" for "oxygen", while English and some romance languages use "sodium" for "natrium" and "potassium" for "kalium", and the French, Greeks and Poles prefer "azote/azot" for "nitrogen".
But for international trade, the official names of the chemical elements both ancient and recent are decided by the International Union of Pure and Applied Chemistry, which has decided on a sort of international English language. That organization has recently prescribed that "aluminium" and "caesium" take the place of the US spellings "aluminum" and "cesium", while the US "sulfur" takes the place of the British "sulphur". But chemicals which are practicable to be sold in bulk within many countries, however, still have national names, and those which do not use the Latin alphabet cannot be expected to use the IUPAC name. According to IUPAC, the full name of an element is not capitalized, even if it is derived from a proper noun such as the elements californium or einsteinium (unless it would be capitalized by some other rule). Isotopes of chemical elements are also uncapitalized if written out: carbon-12 or uranium-235.
In the second half of the twentieth century physics laboratories became able to produce nuclei of chemical elements that have a half life too short for them to remain in any appreciable amounts. These are also named by IUPAC, which generally adopts the name chosen by the discoverer. This can lead to the controversial question of which research group actually discovered an element, a question which delayed the naming of elements with atomic number of 104 and higher for a considerable time. (See element naming controversy).
Precursors of such controversies involved the nationalistic namings of elements in the late nineteenth century. For example, lutetium was named in reference to Paris, France. The Germans were reluctant to relinquish naming rights to the French, often calling it cassiopeium. The British discoverer of niobium originally named it columbium, in reference to the New World. It was used extensively as such by American publications prior to international standardization.
रसायनिक चिंत
विषेश रसायनिक तत्त्व
Before chemistry became a science, alchemists had designed arcane symbols for both metals and common compounds. These were however used as abbreviations in diagrams or procedures; there was no concept of atoms combining to form molecules. With his advances in the atomic theory of matter, John Dalton devised his own simpler symbols, based on circles, which were to be used to depict molecules.
The current system of chemical notation was invented by Berzelius. In this typographical system chemical symbols are not used as mere abbreviations - though each consists of letters of the Latin alphabet - they are symbols intended to be used by peoples of all languages and alphabets. The first of these symbols were intended to be fully universal; since Latin was the common language of science at that time, they were abbreviations based on the Latin names of metals - Fe comes from Ferrum, Ag from Argentum. The symbols were not followed by a period (full stop) as abbreviations were. Later chemical elements were also assigned unique chemical symbols, based on the name of the element, but not necessarily in English. For example, sodium has the chemical symbol 'Na' after the Latin natrium. The same applies to "W" (wolfram) for tungsten, "Hg" (hydrargyrum) for mercury, "K" (kalium) for potassium, "Au" (aurum) for gold, "Pb" (plumbum) for lead, and "Sb" (stibium) for antimony.
Chemical symbols are understood internationally when element names might need to be translated. There are sometimes differences; for example, the Germans have used "J" instead of "I" for iodine, so the character would not be confused with a roman numeral.
The first letter of a chemical symbol is always capitalized, as in the preceding examples, and the subsequent letters, if any, are always lower case (small letters).
साधारण रसायनिक चिं
There are also symbols for series of chemical elements, for comparative formulas. These are one capital letter in length, and the letters are reserved so they are not permitted to be given for the names of specific elements. For example, an "X" is used to indicate a variable group amongst a class of compounds (though usually a halogen), while "R" is used for a radical, meaning a compound structure such as a hydrocarbon chain. The letter "Q" is reserved for "heat" in a chemical reaction. "Y" is also often used as a general chemical symbol, although it is also the symbol of yttrium. "Z" is also frequently used as a general variable group. "L" is used to represent a general ligand in inorganic and organometallic chemistry. "M" is also often used in place of a general metal.
आइसोटोप चिंत
The three main isotopes of the element hydrogen are often written as H for protium, D for deuterium and T for tritium. This is in order to make it easier to use them in chemical equations, as it replaces the need to write out the mass number for each atom. थ्व थे याना च्वै:
D२O (हेभी वाटर)
थे याना च्वइ मखु:
2H२O
मात्रा
During the early phases of the Big Bang, nucleosynthesis of hydrogen nuclei resulted in the production of hydrogen and helium isotopes, as well as very minuscule amounts (on the order of 10-10) of lithium and beryllium. No heavier elements were produced. As a result, the primordial abundance of atoms consisted of roughly 75% 1H, 25% 4He, and 0.01% deuterium.[११] Subsequent enrichment of galactic halos occurred due to Stellar nucleosynthesis and Supernova nucleosynthesis.[१२] However intergalactic space can still closely resemble the
primordial abundance, unless it has been enriched by some means.
The following table shows the ten most common elements in our galaxy (estimated spectroscopically), as measured in parts per million, by mass.[पुष्टि(साइटेसन) मागु] Nearby galaxies that have evolved along similar lines have a corresponding enrichment of elements heavier than hydrogen and helium. The more distant galaxies are being viewed as they appeared in the past, so their abundances of elements appear closer to the primordial mixture. As physical laws and processes appear common throughout the visible universe, however, it is expected that these galaxies will likewise have evolved similar abundances of elements.
| तत्त्व | पार्टस् प्रति मिलियन मास कथं |
|---|---|
हाइड्रोजन | ७३९,००० |
हेलियम | २४०,००० |
अक्सिजन | १०,७०० |
कार्बोन | ४,६०० |
नियोन | १,३४० |
न | १,०४० |
नाइट्रोजन | ९७० |
सिलिकन | ६५० |
म्याग्नेजियम | ५८० |
सल्फर | ४४० |
लिधंसा
↑ Compendium of Chemical Terminology, chemical element
↑ A. Earnshaw, Norman Greenwood. Chemistry of the Elements, Second Edition. Butterworth-Heinemann, 1997
↑ Hillar, Marian (2004). The Problem of the Soul in Aristotle's De anima. NASA WMAP. 2006-08-10 कथं।
↑ Partington, J.R. (1937). A Short History of Chemistry. New York: Dover Publications, Inc..
↑ ५.०५.१ Strathern, Paul. (2000). Mendeleyev’s Dream – the Quest for the Elements. New York: Berkley Books.
↑ Partington, J.R. (1937). A Short History of Chemistry. New York: Dover Publications, Inc.
↑ ७.०७.१ Boyle, Robert (1661). The Sceptical Chymist.
↑ Carey, George, W. (1914). The Chemistry of Human Life.
↑ Sanderson, Katherine (17 October 2006). Heaviest element made - again. nature@news.com. Nature (journal). 2006-10-19 कथं।
↑ Phil Schewe and Ben Stein (17 October 2006). Elements 116 and 118 Are Discovered. Physics News Update. American Institute of Physics. 2006-10-19 कथं।
↑ Wright, Edward L. (September 12, 2004). Big Bang Nucleosynthesis. UCLA Division of Astronomy. 2007-02-22 कथं।
↑ G. Wallerstein,
I. Iben Jr., P. Parker, A. M. Boesgaard, G. M. Hale, A. E. Champagne, C. A. Barnes, F. KM-dppeler, V. V. Smith, R. D. Hoffman, F. X. Timmes, C. Sneden, R.N. Boyd, B.S. Meyer, D.L. Lambert (1999). "Synthesis of the elements in stars: forty years of progress" (pdf). Reviews of Modern Physics 69 (4): 995–1084. Retrieved on 2006-08-04.
स्वयादिसँ
- रसायनिक चिं
- रसायन शास्त्र
- पिरियोडिक टेबल
पिनेया स्वापूत
| विकिमिडिया मंका य् थ्व विषय नाप स्वापु दुगु मिडिया दु: Chemical elements |
Elementymology & Elements Multidict word history and language dictionary- SDV Nuclear Glossary
रसायनिक सूचं
- वेबइलेमेन्टस्
- केमिकूल
- केमिकलइलेमेन्ट
- लस एलामोस देय् प्रयोगशाला
पिरियोडिक टेबल | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||||||
1 | H | He | ||||||||||||||||||||||||||||||
2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||
3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||
4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||
5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||
6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo |
परिमार्जित | ||||||||||||||||||||||||||||||||
| विकिमिडिया मंका य् थ्व विषय नाप स्वापु दुगु मिडिया दु: Chemical elements |
पुचतः:
- पुष्टि(साइटेसन) मदुगु च्वसु
- रसायनिक तत्त्व
(window.RLQ=window.RLQ||[]).push(function()mw.config.set("wgPageParseReport":"limitreport":"cputime":"0.496","walltime":"0.569","ppvisitednodes":"value":19127,"limit":1000000,"ppgeneratednodes":"value":0,"limit":1500000,"postexpandincludesize":"value":87677,"limit":2097152,"templateargumentsize":"value":25226,"limit":2097152,"expansiondepth":"value":15,"limit":40,"expensivefunctioncount":"value":0,"limit":500,"unstrip-depth":"value":0,"limit":20,"unstrip-size":"value":6379,"limit":5000000,"entityaccesscount":"value":0,"limit":400,"timingprofile":["100.00% 419.751 1 -total"," 79.61% 334.166 1 Template:तत्त्व"," 68.46% 287.348 118 Template:Element_cell"," 26.20% 109.980 1298 Template:Trim"," 6.41% 26.888 1 Template:Tnavbar-collapsible"," 5.77% 24.222 1 Template:Navbar"," 4.96% 20.840 2 Template:Commons"," 4.37% 18.350 2 Template:Sister"," 3.62% 15.205 2 Template:Side_box"," 2.63% 11.041 2 Template:Sec_link_auto"],"scribunto":"limitreport-timeusage":"value":"0.005","limit":"10.000","limitreport-memusage":"value":606259,"limit":52428800,"cachereport":"origin":"mw1272","timestamp":"20190410010902","ttl":2592000,"transientcontent":false);mw.config.set("wgBackendResponseTime":733,"wgHostname":"mw1272"););